Indian Medical Devices market to grow to USD 50 billion by 2025
- Sanjay Trivedi
- Feb 19, 2019
- 3 min read
“While India continues to maintain leadership position in manufacturing and supply of high quality generic medicines, the Government’s focus is on improving access to affordable healthcare for all and contribute to the largest Government-funded healthcare programme in the world, Ayushman Bharat”, said Union Minister of Chemicals and Fertilizers, Statistics and Programme Implementation, Shri D.V. Sadananda Gowda, while inaugurating the 4th edition of India’s biggest Global Conference on Pharmaceuticals and Medical Devices, in Bengaluru today. The occasion was also graced by the presence of Union Minister of State for Chemicals & Fertilizers, Road Transport and Highways, Shipping, Shri Mansukh L Mandaviya.

The event is being organized by Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, along with Federation of Indian Chambers of Commerce & Industry (FICCI). The theme of ‘India Pharma 2019’ is ‘Enabling Quality Affordable Healthcare & that of ‘India Medical Device 2019’ is ‘Accelerating Growth to Achieve Universal Healthcare’. This conference is an endeavour of this Government to provide affordable, quality healthcare to citizens of the country and also to encourage ‘Make in India’ while promoting the growth and development of Indian Pharmaceutical and Medical Device Industry.

In his address, Shri Gowda remembered the late Union Cabinet Minister Shri Ananthkumar for his vision to bring all stakeholders of pharma and medical devices industry on one platform through this conference and to help the Government to bring policy changes for removing roadblocks by addressing the pressing issues in this sector. The Government continues to strive to increase the ease of doing business in India through such initiatives.
The Minister said that India continues to maintain its leadership position in manufacturing and supply of high quality generic medicines. With several proactive measures by the Government, exports have improved and the growth in Pharma sector is back on track. The Pharma industry is expected to grow at a CAGR of 15% in the near future. On the other hand, as per industry estimates, the medical devices market is expected to grow to $50 billion by 2025, he added.
Addressing the inaugural session, Shri Mandaviya stressed on the commendable work the Government is doing to ensure affordable healthcare for all through initiatives like Ayushman Bharat. He said that the Government is taking measures to help Indian pharmaceutical sector become a Rs. 3 lakh crore market, making it a global player. The Minister further said that the Government has taken a number of steps to boost the indigenous API industry growth in India. These include constituting a Task Force containing all stakeholders to study the issues of APIs and chart out a strategy to boost their indigenous manufacturing; giving thrust to cluster based development of API industry; in principle approval to 2 API parks, one each in Assam and Andhra Pradesh, among others.

Shri K.J. George, Minster for Large & Medium Industries, Government of Karnataka, while addressing the gathering, highlighted that the State Governments plans to set up a special pharmaceuticals SEZ in Hassan and Pharma Parks in Bengaluru and Mangalore. Dr. Henk Bekedam, WHO Representative to India stated that considering the increased involvement of the Micro, Small & Medium Enterprises in the medical devices manufacturing sector, WHO will provide technical support to the sector to upgrade the manufacturing standards.
Drug regulators participating in the conference include those from over 30 countries including Russia, Kenya, UK, Malaysia, Indonesia, Saudi Arabia & Uzbekistan participated in the event. Besides this, the event also saw participation of drug regulators from 15 states across India. Senior Government officials from Government of India and State Government and Industry stalwarts from Pharmaceutical & Medical Device sector also graced the event.
During the 2-day event, thematic sessions are being held on areas of Artificial Intelligence in Pharma, Import Export issues, Biologicals, Biopharmaceuticals and New Chemical Entities (NCEs) for the Pharmaceutical Sector. For the Medical Device Sector, thematic sessions are focusing on Diagnostics Ecosystem, Ease of Doing Business and Health Technology Assessment (HTA) Framework. Technical workshop by World Health Organization will help in addressing key challenges before the industry and will also provide tremendous business linkages to the participating companies.
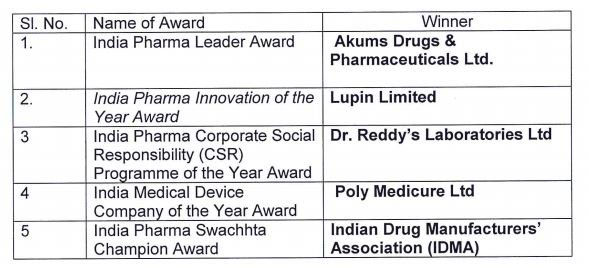
Annual India Pharma & Medical Device awards were conferred by Shri Gowda during the inaugural session for various categories including India Pharma Leader Award, India Pharma Innovation of Year Award, India Pharma Corporate Social Responsibility (CSR) Programme of the Year Award, India Medical Devices Company of the Year Award and India Pharma Swachhta Champion Award. A Knowledge paper on ‘Evolution to Revolution: By Use of Artificial Intelligence & Advanced Analytics in Pharma’ was also released during the session by the Minister.














Comments